Process Validation
Process Validation is conducted to ensure consistent delivery of quality products meeting its predetermined specifications/requirements and quality characteristics. This enables to ensure the complete safety & efficacy of medical device.
IZiel provides complete expertise in process validation and assists medical device manufacturers to consistently meet required parameters through their optimized manufacturing process.
IZiel has successfully delivered various projects through the Onshore-Offshore Work Model thereby completing projects faster and with major cost savings. IZiel team works hand-in-hand with the client to plan, execute, compile reports and receive stakeholder approvals for all process validation projects.
IZiel Approach – Process Verification & Validation
IZiel team makes all efforts to understand the process in detail, investigate the applicable standards and guidelines to determine the process requirements. IZiel’s approach has been very comprehensive and methodical as depicted in the following flowchart.
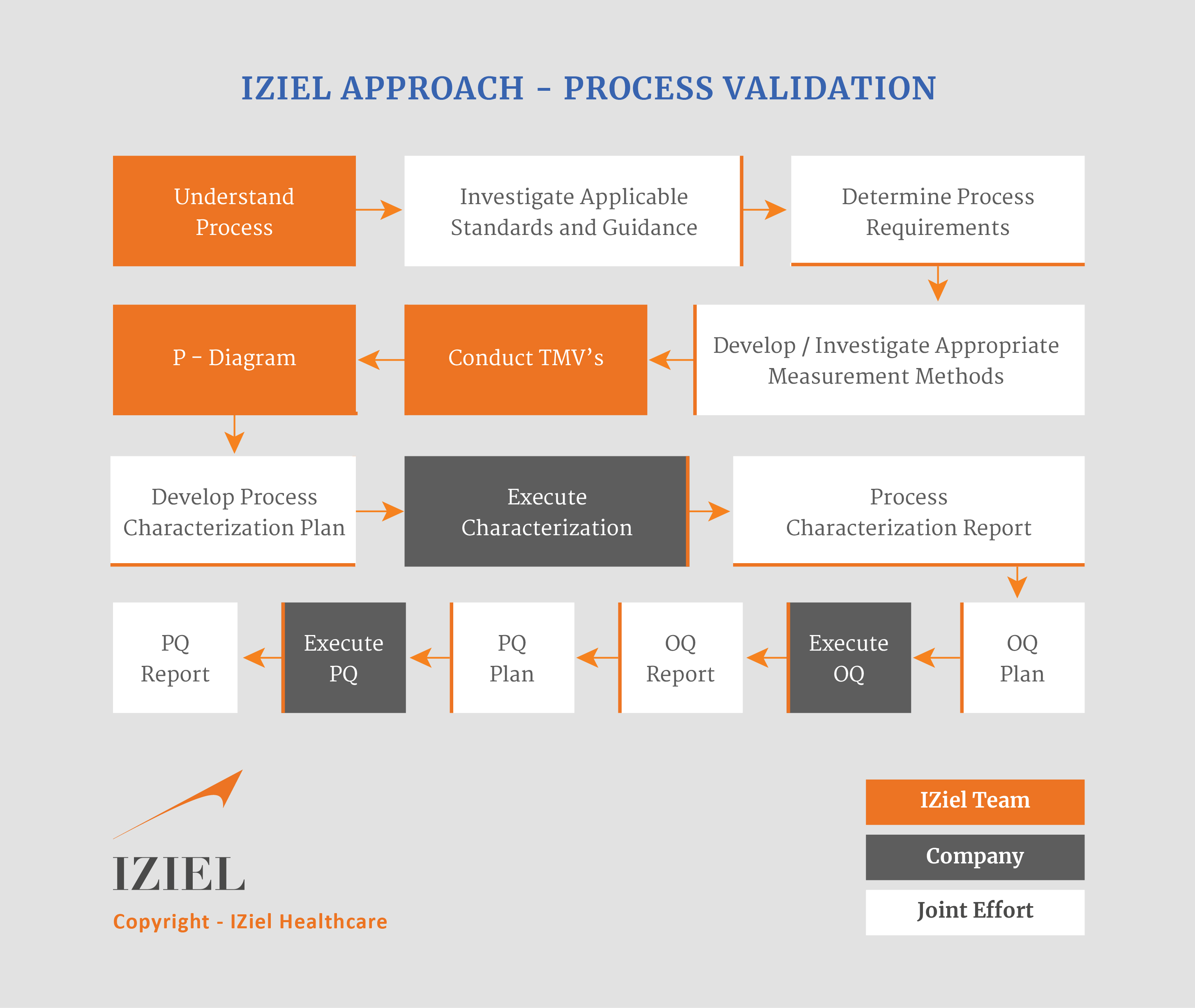
IZiel works with your team to assist you to complete all the following process validation activities
- Process Validation Master Plan (PVMP)
- Define the manufacturing process flow.
- For each process step, define the process requirements.
- For each process requirement, identify the process validation strategy i.e. verification or validation.
- Define equipment, test methods and the relevant validation strategies i.e. IQ and TMV.
- Process Characterization
- Process Characterization is the science of understanding the effects of inputs and other uncontrollable variables on the desired outputs or requirements of a process using robust and proven statistical methodologies.
- Process Characterization is the first step of the Operational Qualification (OQ) activity.
- Extensive use of Transfer Function determination through First Principles or Designed Experimentation is done during Process Characterization.
- Equipment Installation and Qualification (IQ)
- IQ is the task of proving that the equipment is fit for its intended use.
- Requirements for the equipment considering its use and functionality are carefully captured.
- Within a lifecycle approach to process validation, employing a risk based decision making throughout that lifecycle, helps identify critical process parameters throughout the lifecycle as opposed to only certain stages of the process
- The Qualification plan should consider the requirements of use and can incorporate risk management to prioritize certain activities and to identify a level of effort in both the performance and documentation of qualification activities.
- Operational Qualification (OQ) –
- Operational Qualification involves using the results of the Process Characterization studies and determining a well optimized process window.
- IZiel will work with the process experts to conduct Process Characterization and Process Optimization through the use of advanced statistical tools like Designed Experimentation and Response Surface Modelling.
- A Process Window will be defined and the process challenged by conducting runs at the “Worst Case” settings to understand the impact of potentially unintended process input variations on the process outputs.
- The sampling plan, including sampling points, number of samples, and the frequency of sampling for each unit operation and attribute. The number of samples should be adequate to provide sufficient statistical confidence of quality both within a batch and between batches. The confidence level selected can be based on risk analysis as it relates to the attribute under examination. Sampling during this stage should be more extensive than is typical during routine production.
- A high process capability is desired when all OQ data is analysed.
- Performance Qualification (PQ)
- In order to ensure that part to part or lot to lot variation does not impact the outputs of the process, a Performance Qualification (PQ) run is done. 5 lot of input materials or parts are taken and the process nominal setting is challenged with this normally expected lot to lot variations.
- A high process capability is expected out of PQ run data since this represents long term process capability and stability.
- Test Method Development (TMD) & Validation (TMV)
- IZiel can provide support in the development and validation of Test Methods for inspections for both receiving and during in-process manufacturing.
- With a strong statistical background of its team, smooth executing of TMV’s can be achieved.
- Support for fixture design and development will be provided in order to reduce operator to operator variability where ever needed.
IZiel’s Onshore-Offshore Model works with 1 Onsite Member supported by a team of 3-4 engineers from our Technical Center in India. Typically, the data is collected, evaluated and evidence is developed from the process design stage throughout production. Our Model enables the company to complete the project faster and in a cost-effective manner.