Medical Device Design
Medical Product Design in the Medical Device Life-Cycle is a crucial phase to ensure safety and efficacy of the product.
IZiel Healthcare can support you to train and write verifiable, clear and concise, traceable requirements, and conduct requirements management at system level, subsystem level, and component level by understanding your medical device thoroughly. For this, IZiel has collaborated to with Cognition Cockpit, a Boston based company that has developed a Requirements Management Tool and is predominantly used in the medical device industry.
IZiel follows the standard requirement-based approach to prepare the Design History File (DHF), which ensures the completeness of documents. Our team has extensive experience in developing the Design Document as per US FDA 21 CFR 820.30 and ISO 13485:2016 for all classes of the Medical device.
Design Control is an important part of the medical device design & development. Inorder to control the design and development of a medical device, regulations have been developed by regulatory bodies such as USFDA, European Commission and others. These regulations are known as “Design Control.”
Medical Device – Design Verification & Validation
The traditional waterfall diagram is illustrated to understand the design control process.
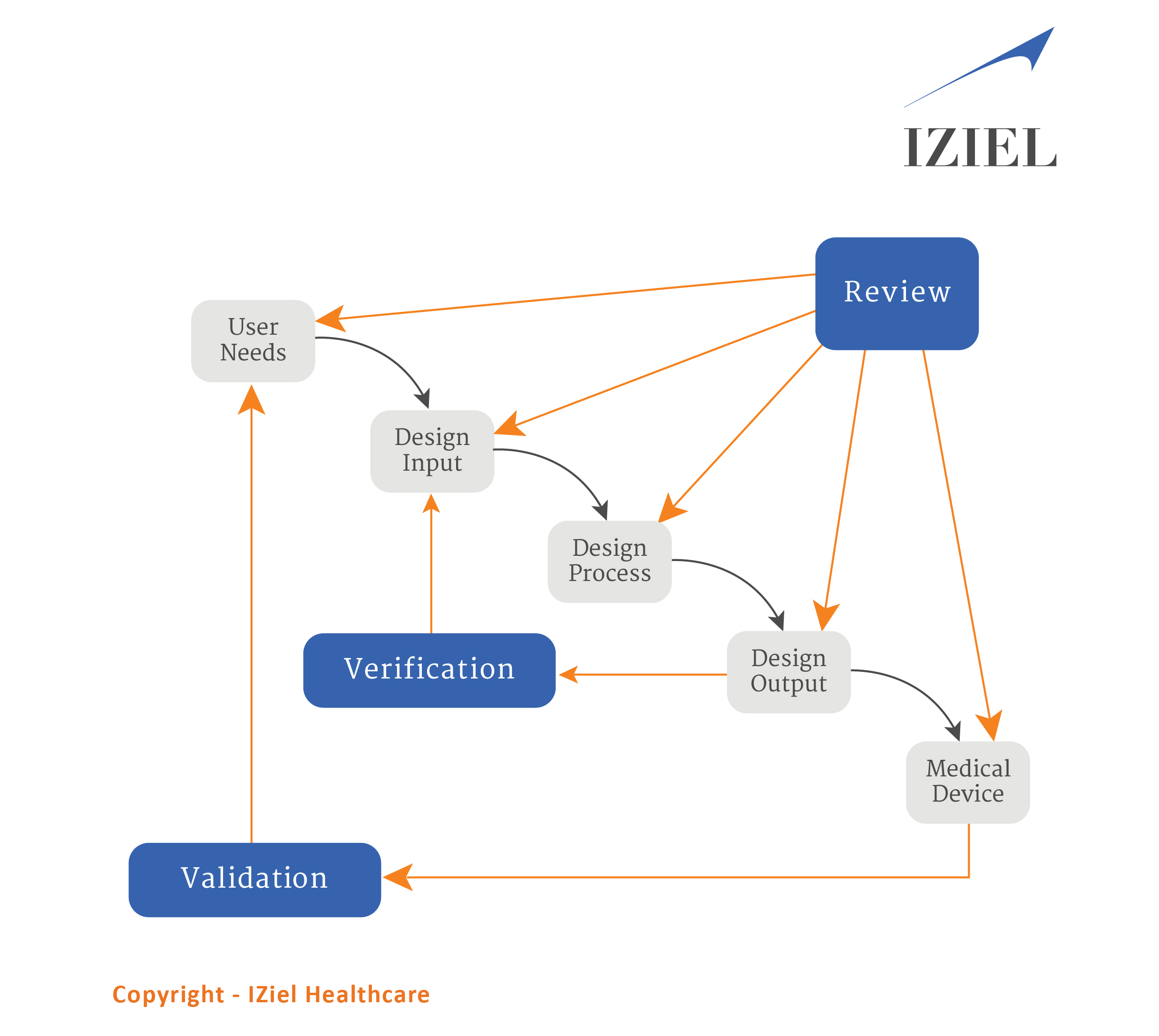
Risk Management identifies, evaluates, analyzes, assesses, and mitigates potential product issues.
When risks are evaluated, risk controls are established to mitigate and reduce risks. In design controls, design outputs, design verifications, and design validations become the risk controls.
- Design Input – Existing safety standards and safety requirements identified in risk assessments are key design inputs.
- Design Output – Risk reduction measures introduced into product design are essential design outputs.
- Design Verification – Design verification should affirm that all safety requirements are covered by the risk reduction measures in the design.
- Design Validation – Design validation should demonstrate that all safety requirements can be consistently met with respect to intended use and user or patient needs.
- Design Transfer – Each scheduled design review should address risk assessment activities, as needed, to ensure that recommended actions are assigned and monitored prior to design transfer.
Why to Choose IZiel Services?
Design control is a set of procedures and practices applied to the design activity intended to ensure that the output product meets the input requirements. It includes understanding user needs, requirements and thereafter design medical device to meet those requirements alongwith risk analysis. Control also includes evaluation of the medical device and transferring it to the production line for manufacturing.
IZiel Healthcare can further support you in preparing complete Design History File (DHF) or Technical File starting from understanding user needs, requirements writing, requirements management, verifying and validating user needs until design transfer.
At IZiel, our team has successfully completed and worked with various client teams to support in developing the Design and Development Plan, Design Verification, Validation Protocol and Reports, Design Transfer Plan and other related documents to complete your design control document.


