QMS Documentation – A Must for Medical Devices
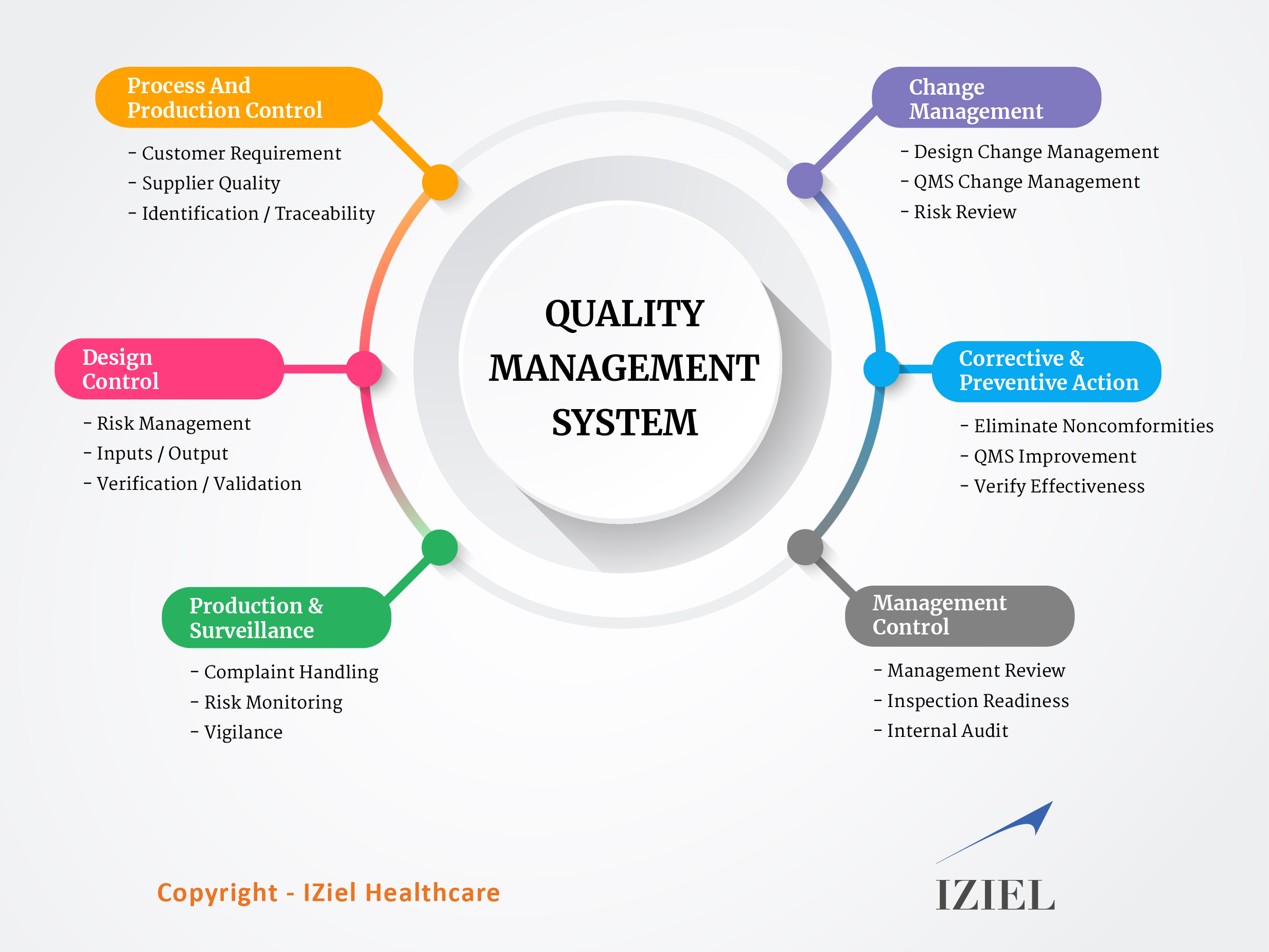
Quality Management System must be designed based on the risk of the product, device classification, complexity of the manufacturing process & size of the manufacturing facility. Irrespective of size of an organization, the Quality Management System consists of the following main sub-systems:
- Management Controls
- Design Controls
- Production & Process Controls
- Corrective & Preventive Action
- Change Management
- Product Surveillance
IZiel Healthcare works with Class I, IIa, IIb & III medical device companies to develop / revamp the QMS to ensure complete product safety & efficacy. IZiel develops comprehensive Quality Management Systems as per USFDA requirements of 21 CFR Part 820, ISO 13485 as well as applicable requirements of MDR 2017/745.
IZiel works with medical device companies to integrate quality & regulatory requirements using a risk-based approach. IZiel has developed their own structured system of procedures, templates, forms and processes covering all aspects of design, manufacturing, risk management, document control, product labelling, supplier management, complaint handling and more.
Implementation of QMS plays a vital role and must be done thoroughly to ensure safety, efficacy & high quality with repeatability. There is a common misconception that quality management system is to be implemented only during the production stage.
However, working with IZiel would ensure that the customer would implement QMS from product development to post-production. QMS Systems such as Design Control, Risk Management, Document Control, Records Management & Supplier Management are implemented in product development stage to ensure smooth transition into the production phase. Robust post-production quality management system must be implemented to enable effective documentation & follow up of customer complaints & feedback.
IZiel Expertise – Complete QMS Documentation Development
Following infographic depicts various sub-systems included in the IZiel QMS –
IZiel works with the Management to train on various responsibilities for implementing and maintaining the QMS as well as works with the Technical & Quality Assurance team complete procedures, forms & templates including how to document and record information.
IZiel covers all the product-specific requirements for all components, manufacturing processes, verification & validation alongwith corrective and preventative actions (CAPA) for assessing customer satisfaction, product non-conformance, assessing and improving quality policies and procedures, carrying out and assessing the results of internal audits, and implementing systems for continuous improvement.
IZiel works with clients to make the QMS simple yet effective and flexible to allow changes in order to keep up with the changing regulatory requirements.