It is the process to generate clinical evidence to establish the safety and efficacy applicable to all the classes of medical devices. The Clinical Evaluation should be drafted with the help of MEDDEV 2.7/1 Rev. 4, using MDCG Guidelines (MDCG 2020-1, MDCG 2020-6, MDCG 2020-13, IMDRF MDCE WG/N56FINAL:2019 and Annex XIV, Article 61 of EU MDR)
The legal manufacturer must draft the Clinical Evaluation Report (CER) in compliance with General Safety and Performance Requirements (GSPR) Checklist. The aim of a CER is to reduce the risk that the users and patients are exposed to when using a medical device.
The steps required for conducting a clinical evaluation:
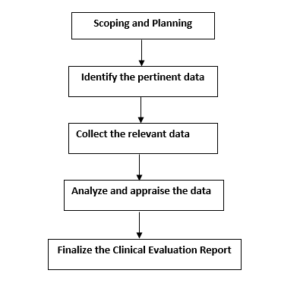
The final Clinical Evaluation Report (CER) will summaries:
- Device description and intended use of the product. The purpose of CER is to collect, appraise and analyze the clinical data obtained from literature searches for the safety and effective performance of the device.
- The text articles obtained from literature searches that includes all the clinical indications, similar devices, clinical benefits, and medical alternatives with the statistical significance. It will also capture the literature search results for the subject device filtered from the defined appraisal criteria. The clinical data constitutes of alternative medical treatment options, risk situations, lifetime, clinical benefits, clinical indications for similar and equivalent devices, adverse event reporting databases, medical device recalls, field safety notice generated, and field safety corrective actions performed for the device, similar, benchmark and equivalent devices.
- The safety and performance of the device mentioning all the risk situations derived from literatures as an input to the risk files, how to apply the appropriate control measures, how to mitigate the risks as far as possible making sure no residual risk remains. If any residual risk exists, it should be mentioned in the Instruction for Use (IFU) as a warning or contraindication.
- Pre-clinical testing performed as per the applicable standards. The compliance of CER with the GSPR requirements is summed up. All the complaints obtained from post-market surveillance is identified for quantitative summation. The post-market clinical follow-up activity, if any, including ethical committee approval, patient consent form, sample size, statistical analysis, side effects or any risks encountered, corrective and preventive actions performed is summarized. The clinical investigation required for any novel device and the Clinical Development Plan is drafted, if any clinical study is performed.
- Finally, conclude CER for safety and effective performance of the device and its compliance with GSPR.
IZiel provides a unique solution developing Clinical Evaluation Plan (CEP), Clinical Evaluation Report (CER) and thereafter provide the physicians certificate. Our partners have a network of 40+ National Board Certified Physicians that conduct the risk-benefit analysis and provide the necessary certification.
To know more about IZiel, please visit our website Who We Are – IZiel
You can contact us at Contact Us – IZiel